Absorbable Gelatin Sponge (medical hemostatic sponge)
- FOB Price:Get Latest Price >
- Min.Order:100 Piece(s)
- Payment Terms:L/C , T/T , D/P , Others
- Favorite
Business Type:Manufacturer
Country/Region:China
Ddu Verified
HOT Rank


Jiangxi Xiangen Medical Technology Development Co., Ltd
We are professional supplier of Absorbent gelatin sponge,Infrared Umbilical Belt,BioActive Umbilical Belt,Umbilical Belt, Absorbable Gelatin Sponge .
Business Type:Manufacturer
Country/Region:China
Ddu Verified
HOT Rank

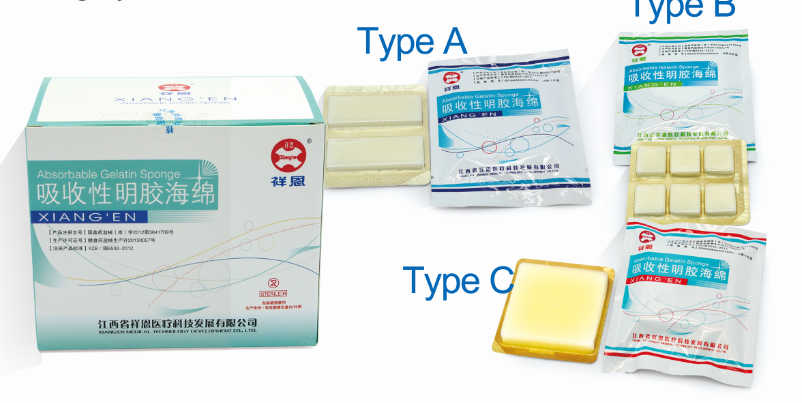

[Product name] Absorbable Gelatin Sponge (medical hemostatic sponge)
[Primary Structure] This product is derived from pig skin as raw material after frothing, curing, drying, sterilization and other processes.
[Bases] This product is mainly made of collagen and the protein content is greater than 85%.
[The scope of application] This product can be applied on wound areas in both first aid and surgeries to stop bleeding.
[Instructions] Product package that is damaged, damped or contaminated should be disposed immediately. Before use, the wound area should be cleaned and disinfected. Removing product from the package should be conducted under sterilized conditions and aseptic manner. This product can be applied in both "drying use" and "saline infiltration" methods to stop bleeding.
Drying use: 1. the product should be cut into proper size and shape. 2. Gently stick the product to the cleaned and disinfected wound area. 3. Impose appropriate pressure on the product until hemostasis. Once stop bleeding, the excess shall be removed.
Saline infiltration: 1. the product should be cut into proper size and shape. 2. This product should be immersed in sterile saline, re-pressing 2-3 times to exclude all air bubbles, and then put aside in the sterile saline for further use. 3. Take the product out from the sterile saline and place it on the sterile gauze to remove excess solution and dry it to the desired humidity. Together with the sterile gauze, gently press the product on the wound area until hemostasis. 4. Use a few drops of saline to moist the gauze, and then remove the gauze. Upon hemostasis, product adhered to the bleeding area should be retained and any excess of the product should be removed.
[Taboos] 1. As this product is collagen products, any application of the products on patients who are allergic to collagen is strictly prohibited. 2. Do not use this product for the blood vessel lumen areas, so as not to cause blood vessel blockage. 3. This product is prohibited to be used for controlling arterial bleeding, it should not be used in the blood or other body fluids collection point. 4. This product is prohibited to be used as adhesion prevention products. 5. This product is sterilized by irradiation. The product should be disposed after use rather than re-sterilization. The product should be taken out in an aseptic manner to prevent contamination before use. Product packaging appears damaged, open, moisture or contaminated, etc. is strictly prohibited to be used further. 6. Do not use this product at skin incision edge; application of such can cause wound edges unable to close properly. This interference is caused by physical mechanical occupying effect caused from gelatin, not other product inherent natures.
[Note] 1. Use the minimum amount of the product to achieve hemostasis purposes. Any excess of this product should be removed and abandoned, so as to absorb or reduce foreign body reaction. 2. When placed into the cavities or closed tissue spaces, light pressure is recommended to be imposed on the product in advance to avoid possible expansion to its original size after absorbing excess body fluids, which might cause damage to the peripheral nerve. 3. Under the circumstances when stop bleeding requires filling the cavity, any excess beyond the need of maintaining hemostasis should be removed. 4. This product cannot be used in conjunction with methyl methacrylate adhesive. Due to collagen fiber may reduce the strength of the methyl methacrylate binder, causing the separation of devices bonded to the surface of the bone by the binder and the bone. 5. This product should not be used in the treatment of coagulation disorders major diseases. 6. This product cannot be rescued with autologous blood circulation simultaneously. 7. When using this product in the bone hole, bone fixation region, spinal cord and optic nerve and chiasm, any excess should be removed after hemostasis. 8. This product cannot be used with silver nitrate or any other corrosive chemicals, due to chemical burn area will affect the absorption of the product. 9. ENT surgery should use this product with caution to ensure that the patient do not inhale the product. 10. In neurosurgery, ophthalmology and urology, safety and efficacy of the product is not confirmed. 11. The safety and efficacy of using this product with drugs such as thrombin, antibiotic solution or powder is not confirmed. 12. The safety and efficacy of using this product in children and pregnant women has not been confirmed.
[Storage] The product should be stored in a dry (relative humidity less than 80%), and temperature controlled at 15-30 ℃ (59-86 ℉) storage room. The product shall not be stored with poisonous, harmful or odorous items.
[Specification] A: 60mm × 20mm × 5mm; B: 20mm × 20mm × 5mm;
C: 60mm × 60mm × 10mm; D: 80mm × 60mm × 5mm
[Sterilization] Sterilization is valid for three years. Please refer to validity on the product package or aluminum foil package.